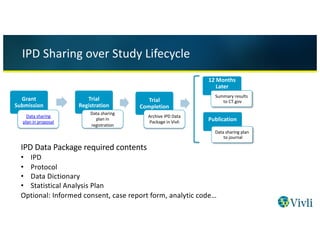
Individual Patient-Level Data Sharing for Continuous Learning: A Strategy for Trial Data Sharing - National Academy of Medicine
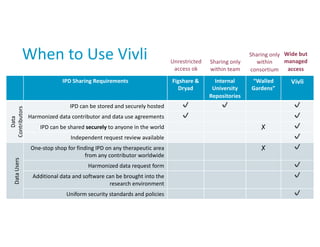
How should individual participant data (IPD) from publicly funded clinical trials be shared? | BMC Medicine | Full Text
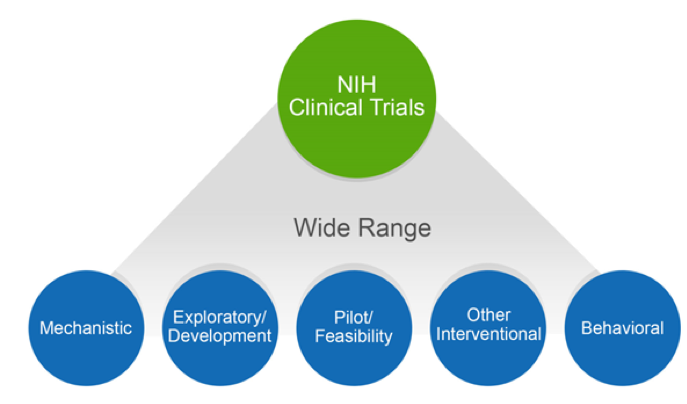
CCTS Provides Tips for Navigating a Changing Clinical Trial Landscape - Center for Clinical and Translational Science | UAB

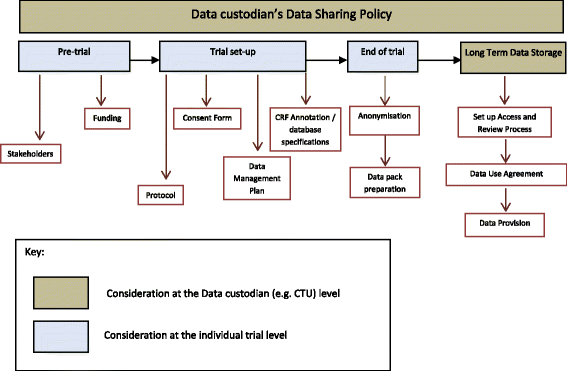
Sharing data from clinical trials: the rationale for a controlled access approach – topic of research paper in Clinical medicine. Download scholarly article PDF and read for free on CyberLeninka open science
Promotion of data sharing needs more than an emergency: An analysis of trends across clinical trials registered on the Internati

Individual Patient-Level Data Sharing for Continuous Learning: A Strategy for Trial Data Sharing - National Academy of Medicine

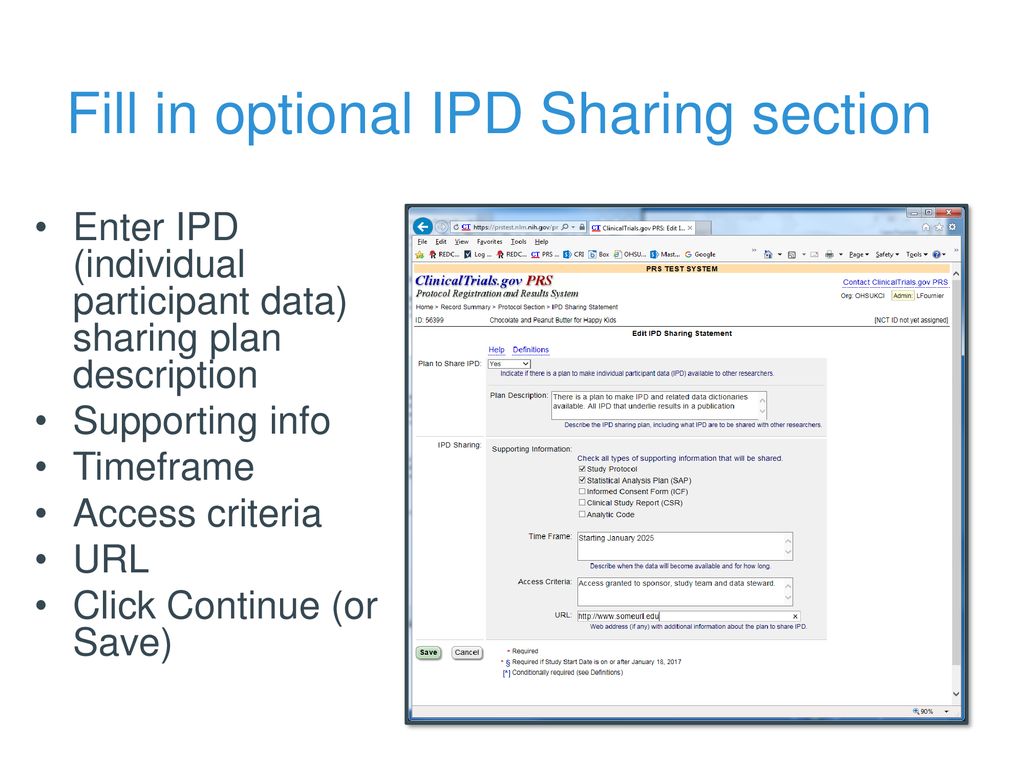
PDSBiggums on Twitter: "$PDSB Interesting new addition to the triple combo study added today. An Individual Participant Data (IPD) Sharing Statement is required in order to have clinical trial results published in

Overview and experience of the YODA Project with clinical trial data sharing after 5 years | Scientific Data